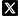Scientists from Fudan University define a neural circuit that regulates giving up, quitting or stopping unsuccessful behavior
In challenging situations, individuals might keep trying to achieve a desired outcome and may even try harder each time. But repeated failure after many attempts often causes individuals to give up, quit and/or stop unsuccessful behavior. How the mammalian brain makes the decision to switch from action to no-action during challenging experiences remains an open question. This area of research is challenged by lack of suitable animal models.
On June 23rd 2023, Dr. Nashat Abumaria research team in collaboration with Dr. Yu Gu team (State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institutes of Brain Science, Fudan University) online published their research results in Neuron, entitled A neural circuit for regulating a behavioral switch in response to prolonged uncontrollability in mice. The study used fast high-resolution miniature two-photon microscopy together with other in vivo recording methods in behaving mice to define the circuit, neurotransmitters and receptors governing a behavioral switch in response to prolonged uncontrollability (Fig. 1)

Fig. 1. Graphical presentation summarizing the study.
This study established two new mouse models relevant to giving up, quitting, and/or stopping unsuccessful behavior by exposing mice to prolonged experiences with an uncontrollable outcome and showing gradual transition in their behavior from action to no-action (Fig. 2). The authors show that this behavioral transition is not caused by pain desensitization, muscle fatigue, anhedonia or learned helplessness.

Fig. 2. Exposure to prolonged uncontrollable experiences such as inescapable discontinuous shock (left) or inescapable discontinuous swimming (right) resulted in behavioral transition from action to no-action.
They used these models to show that noradrenergic neuronal activity in the brain signaled the uncontrollability but did not regulate the behavioral transition. Meanwhile, the release and/or concentration of norepinephrine in orbitofrontal cortex, a brain region involved in adaptive decision-making according to outcome/risk/punishment, regulates the behavioral transition (Fig. 3).

Fig. 3. Norepinephrine neuronal activity remained persistent and increased during exposure to uncontrollable experiences (left). Norepinephrine concentration and/or release in orbitofrontal cortex declined during the behavioral transition from action to no-action (right).
Furthermore, to provide further insights at the cellular level, the authors imaged the real-time activity of GABAergic neurons using miniature two-photon microscopy, reveal that the inhibitory neurons in the orbitofrontal cortex, but not the excitatory neurons or astrocytes, are the major regulators of the behavioral transition (Fig. 4).

Fig. 4. Activation/inhibition of inhibitory neurons in orbitofrontal cortex promoted action/no-action in both models and the behavioral transition from action to no-action is associated with reduction in inhibitory neuronal activity.
Finally, they show that uncontrollable experiences reduce the expression of alpha 1 adrenoreceptors in the orbitofrontal cortex, and that norepinephrine regulates the behavioral transition as well as the activity of inhibitory neurons by alpha 1a receptor (Fig. 5).

Fig. 5. Uncontrollable experience reduced the expression of alpha 1 adrenoreceptors in the orbitofrontal cortex and suppressing the alpha 1a receptor in inhibitory neurons suppressed action behavior.
Therefore, noradrenergic neurons projecting to GABAergic neurons within orbitofrontal cortex are key regulators of adaptive decision making to switch behavior in response to prolonged uncontrollability. Reduction of norepinephrine and downregulation of alpha 1 receptor in orbitofrontal cortex results in reduction of the number and activity of inhibitory neurons necessary for driving action behavior leading to behavioral transition to no-action (Fig. 1).
The study generated and successfully validated two new experimental mouse models that differed from endophenotypes associated with other models known in the field of emotions and psychiatric disorders. The findings provide insight into the mechanisms underlying adaptive decision making to give-up, quit or switch behavior in the face of repeated failures. The new paradigms provide tractable models that will help expanding the boundaries of negative emotions for future investigations by the field.
Dr. Chaoqun Li, post doc from Dr. Abumaria lab, and Tianping Sun, Yimu Zhang, Ph.D. students from Institutes of Brain Science, Fudan University, are co-first authors of the paper. Dr. Nashat Abumaria (那德) and Dr. Yu Gu (顾宇) from Institutes of Brain Science, Fudan University, are co-corresponding authors of the paper. Dr. Minmin Luo from Tsinghua University, Dr. Zilong Qiu from Shanghai Jiao Tong University, Dr. Miao He, Dr. Jiayi Zhang and Dr. Lei Xiao from Fudan University provided different Cre-lines and AAV vectors for the current study. Dr. Yi Rao from Peking University provided TPH2+/- mice for the current study. The work was supported by the National Natural Science Foundation (NSF) of China, National Postdoctoral Program for Innovation Talents and Shanghai Municipal Science and Technology Major Project, ZJ Lab, and Shanghai Center for Brain Science and Brain-Inspired Technology.
The miniature two-photon microscopy is provided by Transcend Vivoscope.
For more information, please contact info@tvscope.cn
Information
Related products
No relevant product information