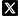Fluorescence imaging microscopy is a powerful tool in biological research and medical diagnostics. It works by using specific wavelength excitation light to stimulate fluorophores in a sample, then capturing the fluorescence signals through a microscope or camera. This technique provides detailed images of cellular structures and proteins.

Principles of Fluorescence Imaging Microscopy:
Fluorophores are molecules that absorb light at one wavelength (excitation) and emit light at a longer wavelength (emission). When excited by light, electrons in the fluorophore molecules jump to a higher energy state and then fall back to a lower energy state, emitting photons that form the fluorescence signal. This shift in wavelength between absorbed and emitted light is known as the Stokes shift.
Widefield Fluorescence Microscopy:
In widefield fluorescence microscopy, the whole field of view is lit up, and the emitted fluorescence is captured simultaneously, facilitating rapid imaging of large areas. It's great for 2D imaging of cell populations, individual cell structures, and specific proteins, and especially useful for live-cell imaging to observe dynamic processes like neuronal signal transmission.
Components include a light source like an LED, which is cost-effective, long-lasting, generates little heat, and needs less alignment compared to traditional mercury arc lamps. The excitation light goes through an excitation filter and reflects off a dichroic mirror into the objective lens to focus on the sample. The fluorophores in the sample emit fluorescence that is collected by the same lens and passes through the dichroic mirror and an emission filter to the detector. For imaging, the fluorescence can be seen directly through an eyepiece or captured by a camera with CCD or CMOS sensors, selected based on frame rate, noise, and sensitivity requirements.
Applications:
 |
 |
| Widefield fluorescence imaging: GCaMPs Calcium imaging | Widefield fluorescence imaging: Microglia imaging |
Cell and Tissue Imaging:Widefield fluorescence microscopy is widely used for visualizing specific cellular structures and proteins within cells. By labeling samples with different fluorophores, researchers can generate multi-color images, highlighting various components of a cell or tissue. For example, green fluorescent protein (GFP) can be used to label actin filaments, while DAPI can stain DNA in cell nuclei.
Live-Cell Imaging: This technique is particularly advantageous for studying dynamic processes in live cells. It allows researchers to track real-time changes, such as the movement of organelles, intracellular signaling, and the interactions between proteins.
Medical Diagnostics: In medical diagnostics, fluorescence imaging microscopy helps in identifying and analyzing pathological changes at the cellular level. It is instrumental in cancer research, where it aids in the detection of specific cancer markers.
Advantages:
High Resolution: Capable of producing high-resolution images.
Fast Imaging Speed: Suitable for capturing dynamic processes in real-time.
Simplicity and Cost-Effectiveness: Relatively straightforward setup and lower cost compared to more complex imaging systems.
Future Directions:
To address the limitations of widefield fluorescence microscopy, advanced techniques such as multiphoton microscopy, structured illumination microscopy (SIM), and deconvolution algorithms are being developed. These methods enhance depth resolution and image clarity, allowing for more detailed three-dimensional imaging of thicker samples.
References:
[1] Manz W , Arp G , Schumann-Kindel G , et al. Widefield deconvolution epifluorescence microscopy combined with fluorescence in situ hybridization reveals the spatial arrangement of bacteria in sponge tissue[J]. Journal of Microbiological Methods, 2000, 40(2):125-134.
[2] Gustafsson M . Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(37):p. 13081-13086.
[3] Zipfel W R , Williams R M , Webb W W . Nonlinear magic: multiphoton microscopy in the biosciences[J]. Nature Biotechnology, 2003, 21(11):1369-1377.
[4] https://www.scientifica.uk.com/learning-zone/widefield-fluorescence-microscopy
[5] https://ibidi.com/content/215-widefield-fluorescence